When mistakes are made picking, packing and shipping products, the inconvenience to your customer is not the only consequence. When it comes to industries such as pharmaceutical distribution, these mistakes can put your customer’s safety at risk and increase the risk of product theft and tampering. Although there are many regulations put forth by the DEA, FDA and DSCSA to protect customers and the general public from these types of concerns, it still requires that your pharmaceutical distribution business take the necessary steps internally to ensure a secure supply chain. One option to better manage inventory is with the use of barcode scanning.
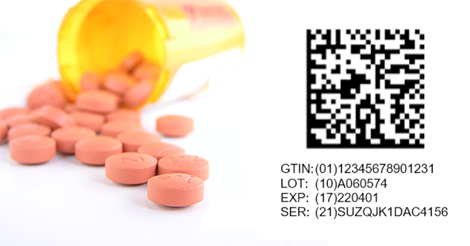
Barcode scanning allows you to track inventory as it moves through your warehouse - from receipt, to picking and packing. In its simplest form, barcodes exist to allow your business to track product movement internally and throughout the supply chain, however, in the pharmaceutical industry important information is also tracked via barcode. Unlike widgets, pharmaceutical products include various properties such as lot number, expiry date, dosage, product family, NDC#, GTIN, strength, schedule, limit and more. Recent regulations mandate that all pharmaceutical products have a 2D datamatrix barcode containing the following four data elements on each product’s smallest unit for sale.
GTIN
The GTIN or Global Trade Item Number is a 14-digit GS1 approved identification key for each trade item. The key includes a GS1 company prefix followed by an Item Reference Number and a check digit.
Lot Number
A lot number is a unique identifier assigned to a single production run of a product.
Serial Number
A serial number is a unique code assigned to a individual item or the lowest sellable unit. For example, you see a unique serial number of each Tylenol bottle but a few Tylenol bottles that were manufactured at the same time, will share a lot number.
To learn more about Lot and Serial number tracking, check out this blog: Understanding Lot and Serial Number Tracking (bluelinkerp.com)
Expiry Date
It's important that Pharmaceutical Distributors follow a FIFO method when shipping out products because many prescription and non-prescription drugs include expiration dates.
This information can then be “parsed” using barcode scanners depending on the specific function being performed. With Blue Link’s pharmaceutical distribution software, the parsed information updates within the software’s database so that important information about each inventory item is easily accessible.
Benefits of Tracking Lot Numbers with Barcode Scanning
There are many benefits to barcode scanning in general, and even more so when managing potentially harmful product such as pharmaceutical inventory.
- By implementing barcode scanning within your warehouse operations, your processes are fully auditable and pharmaceutical products are traceable. This helps deter theft and reduce loss and liability.
- Blue Link’s scanning functionality prevents the need for manually keying in important information such as lot and lot expiry. This not only allows product to be received more quickly into your warehouse and inventory system, it also reduces data entry errors from having to do this manually.
- Pharmaceutical barcodes are also used for saleable returns verification (a DSCSA November 2019 requirement). This functionality allows employees to scan a product’s barcode and using “VRS” immediately check/verify the associated barcode to determine if it is safe to receive back into inventory for resale. Without verification of a product, the item cannot be resold.
Implementing the right Pharmaceutical Distribution Software with barcode scanning capabilities can help to speed up your warehouse process and help to provide a seamless growth transition.
“Blue Link’s ability to populate the lot and expiry date by scanning pharma 2D barcodes is really great news for pharmaceutical distributors. It has huge implications for improving efficiency and reducing errors when receiving pharma products.”
– Michael Benedick, Pharmaceutical Software Expert