The “Drug Supply Chain Security Act (DSCSA)” was enacted by Congress on November 27, 2013 and outlines steps to achieve interoperable, electronic tracing of products at the package level to identify and trace certain prescription drugs as they are distributed in the United States. It is meant to enhance the FDA’s ability to help protect consumers from exposure to drugs that may be counterfeit, stolen, contaminated, or otherwise harmful. The “Act” has many requirements identified for the Pharmaceutical Industry (Manufacturers, Re-Packagers, 3PL’s, Distributors, and Dispenser’s) that must be followed in order to comply with the DSCSA.
To put it into context, as written on the FDA website, the DSCSA consists of 16,690 words not including the tens of thousands (maybe hundreds of thousands) of words written in many Guidance documents to help guide the industry in the development of the various processes.
In order for the industry to develop processes and technology to comply with the requirements set out by the FDA/DSCSA, a period of 10 years was given for all to be implemented. It’s not uncommon to procrastinate when given long deadlines. But with November 2023 fast approaching, in the Pharmaceutical Industry, its crunch time!
August 2023 Update
The FDA has released a guidance delaying the enforcement of the DSCSA requirements until November 27th, 2024. It is not advised to stop or delay implementing interoperable processes, instead this time should be used to perfect the systems and processes.
Read the Guidance: Enhanced Drug Distribution Security Requirements Under Section 582(g)(1) of the Federal Food, Drug, and Cosmetic Act--Compliance Policies | FDA
Although we will not be going into details for every requirement, a walk back in history and what’s in store for the future is worth exploring. The content provided is an overview and does not reflect the detail as written in the DSCSA.
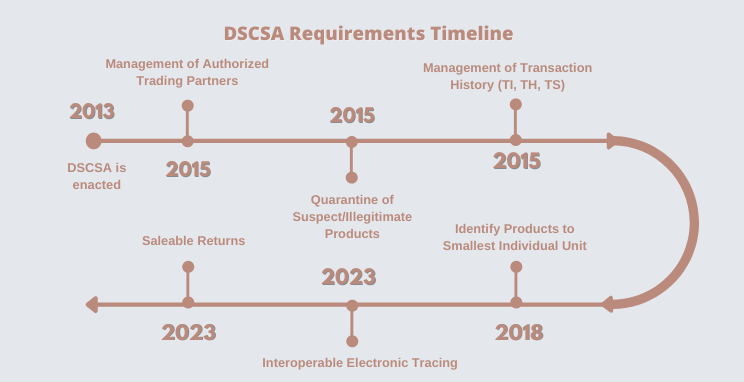
DSCSA Requirements
Here’s our take on the DSCSA requirements and how Pharmaceutical ERP Wholesale Software can help.
November 27, 2013 – President Barack Obama signed DSCSA into Law
2015 – Ensure Pharmaceutical Trading Partners are “Legitimate/Active” - As part of this process valid licenses are now required to be maintained for all suppliers and customers you are doing business with. This is often in the form of a hard copy, but some ERP solutions (such as Blue Link) allow file copies to be stored along with other information for easy access.
2015 – Quarantine Suspect/Illegitimate Products – Drugs have always been lot controlled with expiration dates, but this requires the service provider to physically look at the received product, its packaging, supplier, etc. to ensure there is nothing suspicious about the products being received. If the receiver feels there is something suspicious, a special form - FDA 3911 - must be completed and submitted. The product must then be quarantined (to ensure it is not shipped to other trading partners downstream) until a full investigation is done and the product is no longer considered suspect.
2015 – Product Tracking – Prior to this requirement, lot tracking was at best one up and one down. Now, any service provider in the supply chain are required to know where every product lot came from - starting from the manufacturer and downstream to their customer. A company receiving product(s) cannot ship them until a “Transaction Document (T3)” indicating all “owners” of the product upstream back to the manufacturer or re-packager is provided. This document can be electronic or in hard copy. The T3 for each product sold must include (TI) Transaction Information, (TH) Transaction History, and (TS) Transaction Statement. Many companies try to manage this manually or are using 3rd party solutions that are not integrated to their accounting solution causing delays in sending of the T3 (if they are able to send it at all). Solutions such as Blue Link Pharmaceutical ERP have this functionality integrated so there is no need for manually creating T3’s.
2018 – Product Identification to Smallest Salable Unit – As part of the attempt to prevent illegitimate products from entering the supply chain, the DSCSA now requires all products to have a Product Identifier that includes the NDC, Lot Number, Expiration Date, and a unique Serial Number to the smallest sellable unit. The use of barcode scanners and the 2D barcode has made it more efficient for receiving and picking products and tracking this information.
2019 – Saleable Returns – A requirement that was supposed to be achieved in 2018, then pushed to 2019 and then to 2023 is the ability to authenticate a product that is returned for resale with the manufacturer before it can be resold. Verification of a returned product can be performed in 2 ways: (1) by sending a manual request to the manufacturer or (2) by using a Verification Routing System “VRS” which electronically shares and verifies the information. More advanced inventory and accounting ERP systems have developed functionality that integrates with many of the developed VRS solutions in the market.
2023 – Electronic Interoperability Tracing – Probably the most discussed functionality in the industry to date is the ability to have the electronic means for the entire supply chain to receive information about the shipments they will be receiving from their trading partner to ensure there is no product getting into the supply chain that does not belong there. In short, companies will need the ability to receive electronic data using EPCIS (Electronic Product Code Information Services) formats and verify that the products being received (to the serial number) are what has been shipped by the upstream supplier. In turn, when an order is shipped to the downstream “customer” a file will need to be sent with all the pertinent information for the customer to receive and verify they are receiving the correct product. It is still unclear if this requirement will be enforced starting on November 27, 2023, but the industry is working diligently to make it happen.
Pharmaceutical ERP Software
As a pharmaceutical wholesale distributor, it is imperative that you have the software and systems in place to manage the existing DSCSA requirements (and any future changes). While some of the requirements laid out by the DSCSA can be handled manually, this is only true for small businesses with a low number of inventory items and transactions. As your company grows, you will want to find and work with a software solution that not only provides all the functionality currently required, but is also an active member of the pharmaceutical community to develop potential future requirement features. If you're in the market for a new ERP solution, consider these other features that are important to the pharma industry:
- Short-Dated Lot Management – If you are a company that often finds yourself with short-dated lots, having the ability to manage them could be beneficial.
- Suspicious Order Monitoring (SOM) – If you are selling products that require you to ensure your customer is not purchasing products outside their normal/acceptable patterns, SOM management should be seriously considered as a top priority as not being able to manage this could get your company in trouble with the DEA.
Download our Pharmaceutical Distribution Software Buying Guide for more information on pharma specific features and functionality available in Blue Link ERP.
The DSCSA was enacted to ensure the safety of those using pharmaceutical products and to help prevent illegitimate products from entering the pharmaceutical supply chain. As we get closer to the 2023 deadline make sure that your Pharmaceutical Wholesale Distribution company will be able to comply with all the requirements above and more.