In today's rapidly evolving pharmaceutical landscape, staying compliant with Drug Enforcement Administration (DEA) regulations is more crucial than ever.
The DEA’s Diversion Control Division notes:
“Under federal law, all businesses that import, export, manufacture, or distribute controlled substances; all health professionals licensed to dispense, administer, or prescribe them; and all pharmacies authorized to fill prescriptions must register with the DEA.”
The DEA has mandated that pharmaceutical "registrants" diligently monitor sales orders and promptly report any suspicious activity involving controlled substances to their DEA Field Division Office of the Administration in their area when discovered by the registrant. (See 1301.74 (b)) These suspicious orders can take various forms, such as orders of unusual size, significant deviations from regular purchasing patterns, or orders occurring with uncommon frequency.
Given the complexity of managing multiple customers and ship-to locations, manually policing the order entry process has become an impractical task. To ensure strict adherence to DEA guidelines, your business needs a cutting-edge software solution that can efficiently track, monitor, and report on any potentially suspicious orders. By leveraging advanced technology, you can streamline your compliance efforts, safeguard your operations, and maintain the highest standards of integrity in the pharmaceutical industry.
A DEA FAQ confirms:
Given the complexity of managing multiple customers and ship-to locations, manually policing the order entry process has become an impractical task. To ensure strict adherence to DEA guidelines, your business needs a cutting-edge software solution that can efficiently track, monitor, and report on any potentially suspicious orders. By leveraging advanced technology, you can streamline your compliance efforts, safeguard your operations, and maintain the highest standards of integrity in the pharmaceutical industry.
Blue Link Pharmaceutical ERP – Suspicious Order Monitoring Functionality
With input from our clients and following industry regulations, Blue Link’s complete end-to-end Pharmaceutical Distribution Software includes sophisticated and configurable Suspicious Order Monitoring functionality.
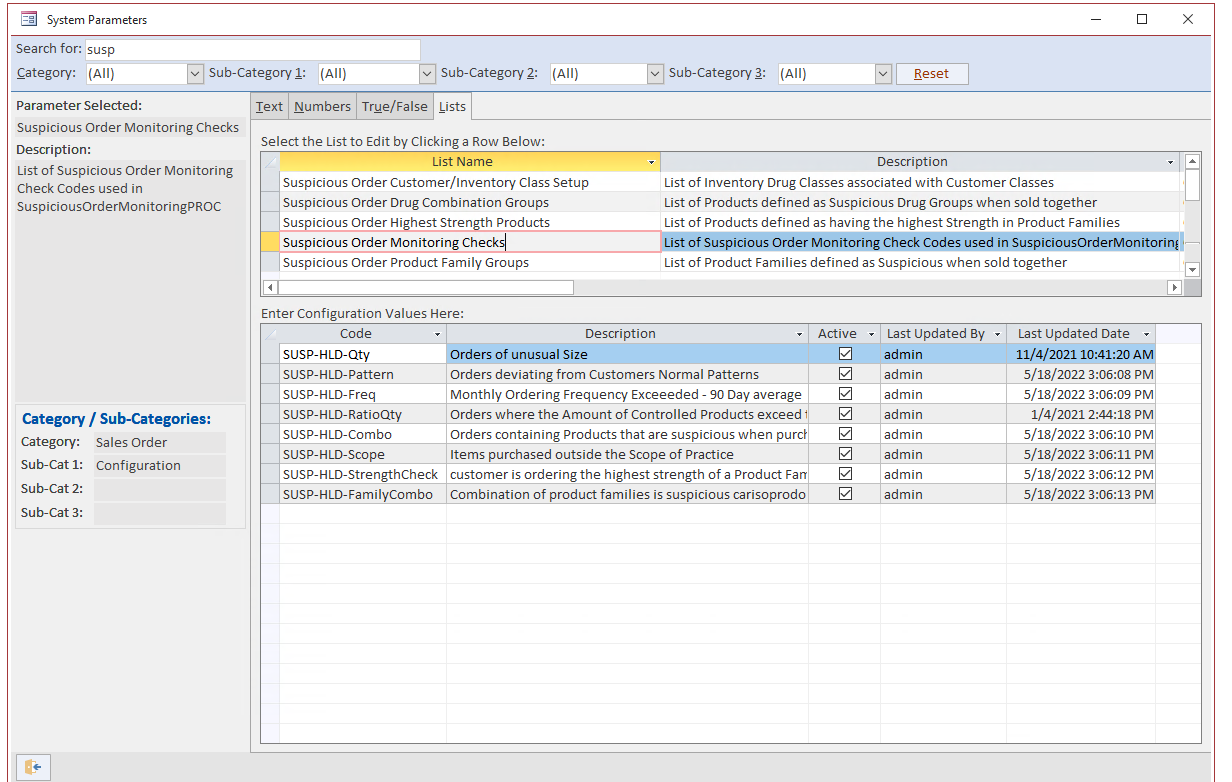
Blue Link’s suspicious order monitoring (SOM) functionality works by monitoring sales order activity in several areas throughout the software. In addition to the criteria listed above and outlined by the DEA, Blue Link allows you to automatically check all sales order activity for a variety of other criteria such as:
- The ratio of controlled vs. non-controlled quantities
- Abnormal combinations of products
- Items outside of the scope of practice
- Strength check
- Different product family combinations
- Orders with a large percentage of products from the same family
Convenience at your fingertips
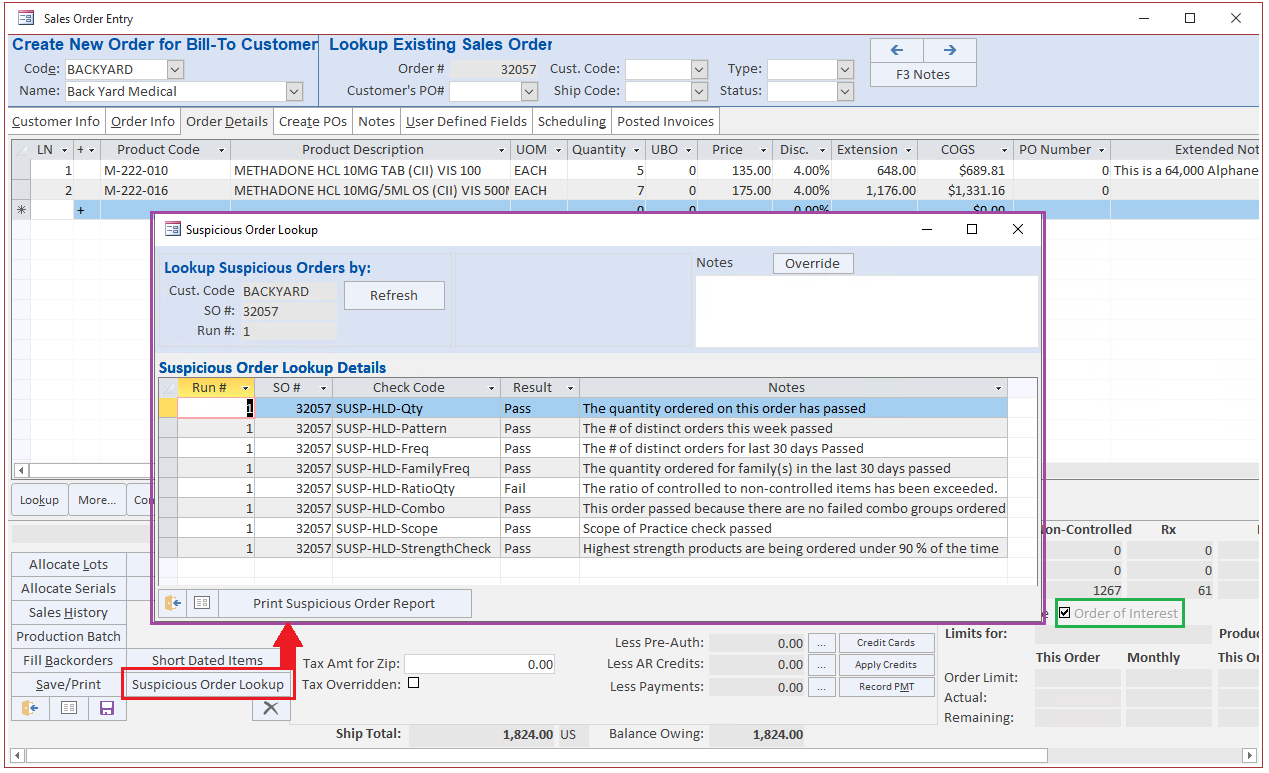
Blue Link provides complete flexibility to our users by allowing you to create additional criteria for SOM outside of those set above. The software also allows you to dictate which products you want to check. Any order that meets the criteria outlined in Blue Link’s SOM tool gets flagged and the system will automatically put the order on hold. An authorized individual will be notified and with the right user permissions, the individual can modify monitoring criteria and release the hold status.
In the event of a customer attempting to purchase an unsuitable product or an error in order quantity input by your team, our system will promptly flag and hold the sales order. This feature not only simplifies new employee training but also provides management with peace of mind by averting unauthorized actions, errors, and violations of DEA and DSCSA regulations.
If a DEA Auditor makes an unexpected visit to your facility.
If a DEA Auditor shows up there is a high chance they will ask the following question:
"Do you have a system in place to monitor suspicious orders?"
If you answer 'yes' they may move on but in the case that they require proof, with Blue Link ERP, you would easily be able to point to the "enabled" SOM checks, and to the history of overrides of those checks with the notes entered at the time the override was done.
With Blue Link’s SOM functionality, you can confidently ensure that all sales orders processed adhere to DEA guidelines and it can help you achieve NABP accreditation, comply with the DEA requirement and show visiting DEA inspectors that you are serious about your responsibilities.