Since 2013, the pharmaceutical supply chain has been working to implement various initiatives described by the Drug Supply Chain Security Act (DSCSA). The DSCSA was enacted by US Congress to help achieve interoperable, electronic tracing of products at the package level to identify and trace certain prescription drugs as they are distributed in the United States. While there have been various requirements outlined over the past 10 years, 2023 marks the deadline for electronic interoperability tracing and the need for pharma serialization software.
DSCSA Requirements
As of November 23, 2023, the DSCSA requires that all members of the pharmaceutical supply chain be able to electronically receive information about shipments of inventory to verify the product received was what was shipped by the supplier and to ensure that illicit product is not getting into the supply chain. This requirement will be managed through the electronic exchange of data following the GS1 International Standard known as EPCIS (Electronic Product Code Information Services).
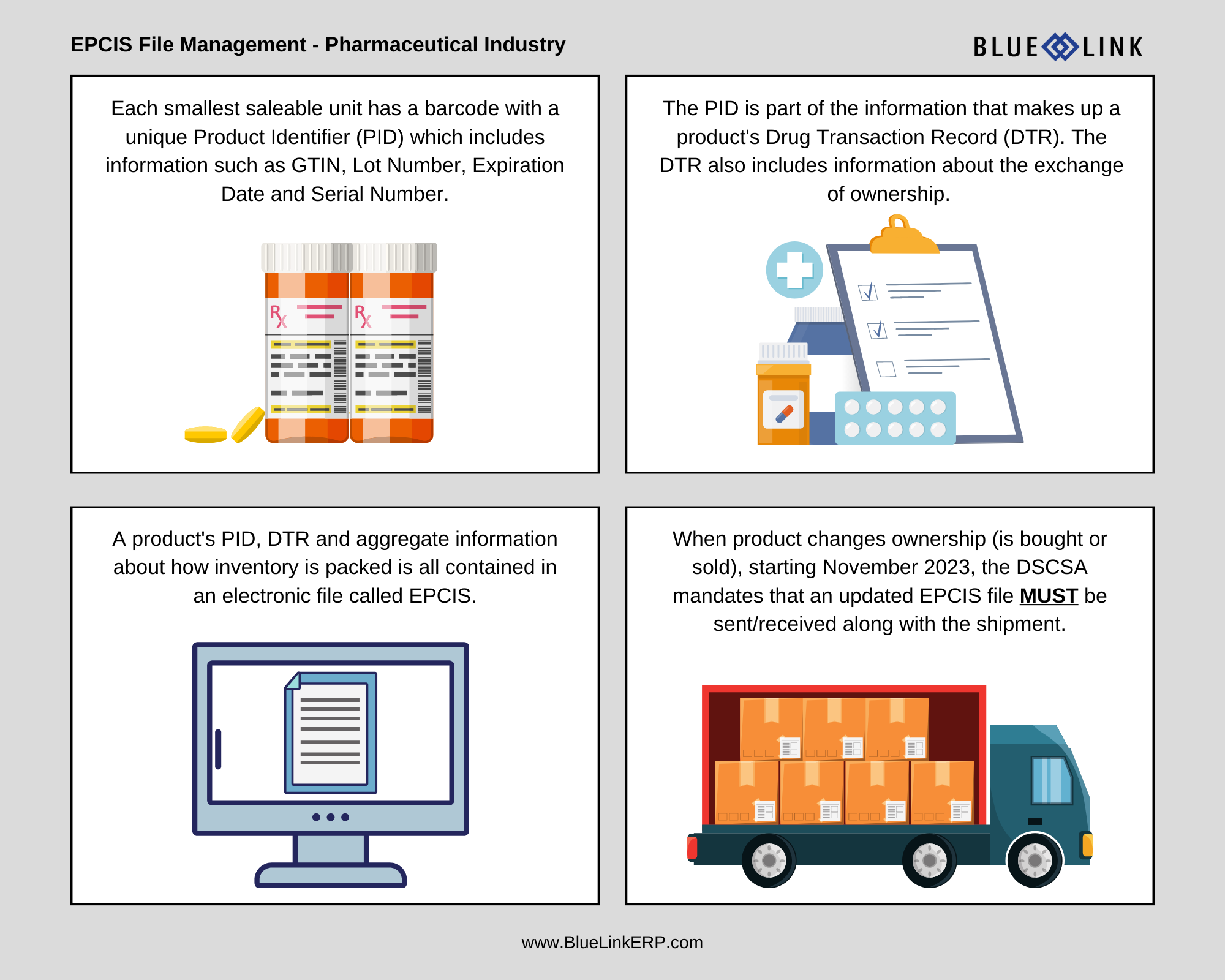
Essentially, trading partners in the supply chain are required to receive, verify and share information about each product as it exchanges ownership. This information includes a unique Product Identifier “PID” (GTIN, Lot Number, Expiration Date, Serial Number) for each saleable unit, which is part of the information that makes up a product’s Drug Transaction Record (DTR).
Managing Pharma Inventory
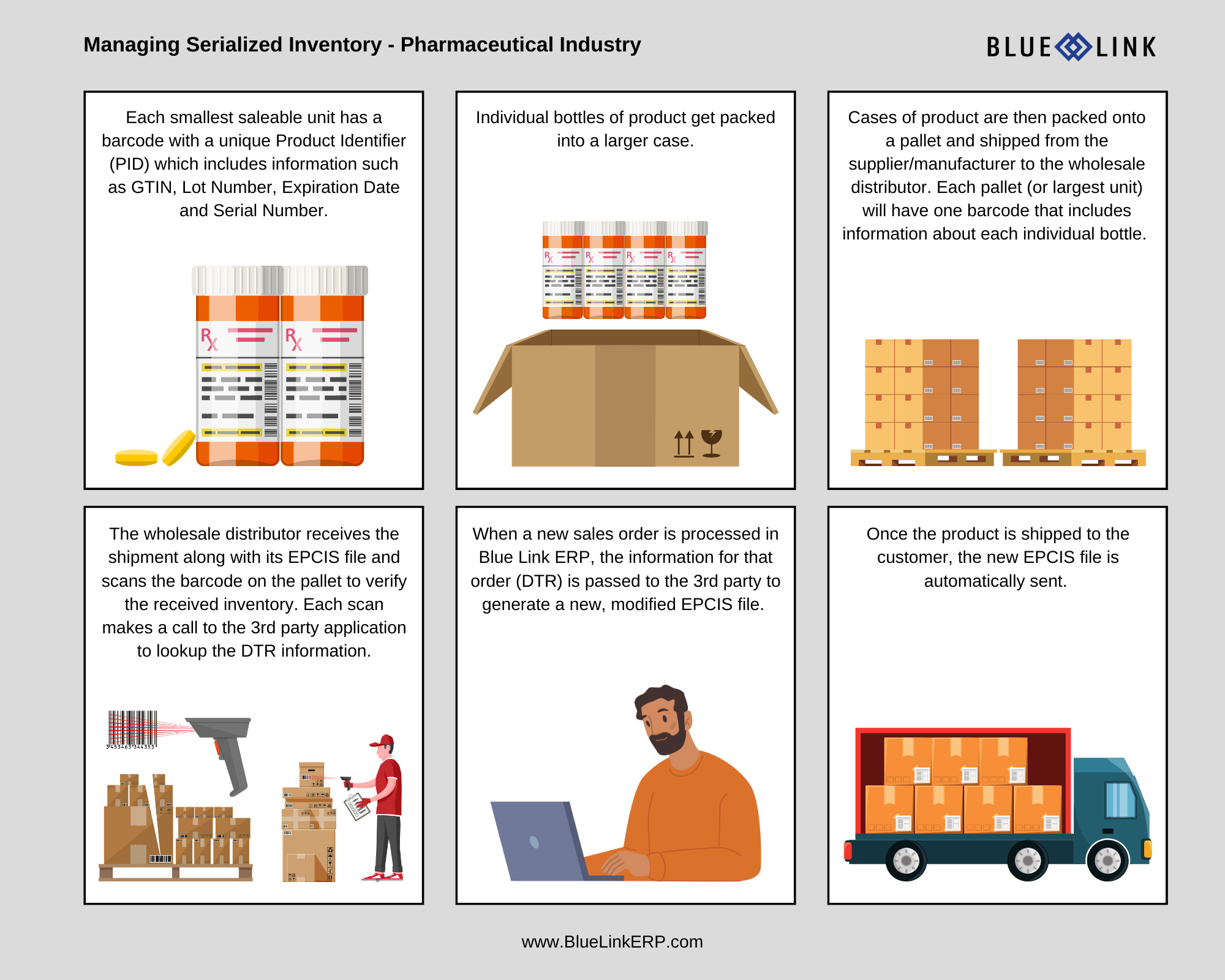
As part of the DSCSA mandate going into effect November 2023, each smallest saleable unit of product must have a serial number. The DSCSA then requires a secure means to track the serial number throughout the entire supply chain (done through the electronic transmission of the EPCIS file). The information within the EPCIS file that gets transmitted between trading partners includes:
- The DTR (which also includes information about the change of ownership)
- Aggregate information on how items are packed (for example, whether a shipment includes eaches in inner packs, inner packs in cases, cases on pallets, pallets in a container, etc.)
The DSCSA requires that an EPCIS file is shared between trading partners with any change in product ownership. The use of EPCIS files, aggregation and inference information allows trading partners to verify the legitimacy of product and makes the receiving process much more efficient. This is because it allows a scan of a sealed case to infer the contents within, as opposed to having to manually scan each individual unit.
What is Pharma Serialization Software?
To best manage the exchange of serialized information and EPCIS files, you need Pharma Serialization Software such as Blue Link. Pharma Serialization Software helps businesses comply with regulations put forth by the DEA, FDA and DSCSA, and includes specific functionality for managing serialized products and the transmission of EPCIS files. Blue Link’s Pharma Serialization Software works best for pharmaceutical wholesalers and distributors in the middle of the supply chain and includes key functionality for managing all business operations including inventory, accounting, order entry, purchasing, warehouse management, contact management and more. These features are necessary for any wholesale distribution business for running day-to-day operations. These features work together with Blue Link’s serialization functionality and other pharmaceutical-specific features such as:
How Blue Link ERP Manages the Electronic Exchange of EPCIS Files
When a Blue Link customer receives a shipment of inventory, they must compare the physical product receipts against the electronic information sent by the supplier (EPCIS) as part of the 2023 requirements. While this can technically either be done manually or electronically, some major suppliers require that all their customers be able to receive EPCIS files electronically. To properly store and process EPCIS file information, Blue Link ERP works with 3rd party applications that can read, decipher, and transmit the information to Blue Link’s ERP software for use in the receiving and shipping process.
When receiving inventory into your warehouse, you’re able to scan the product (largest unit size) and as part of the scan, Blue Link will automatically make a call to the 3rd party application to lookup the DTR information (which is part of the EPCIS file) to verify the product being received. The information is then passed into Blue Link and saved for use in the shipping process.
If the system does not find DTR information associated with the inventory items, an error message will appear, and you will have to set aside the inventory and reach out to your supplier to get the correct information.
When shipping products to your customers, Blue Link allows you to automatically send the EPCIS file to the customer. Every time you scan a product that is associated with the specific sales order, the system will find the information stored in Blue Link, along with other applicable information (customer information, shipping location, etc.). Then, after the shipping process is complete, Blue Link sends the DTR information to the 3rd party to compile into a modified EPCIS file. Once the EPCIS file has been updated, you can automatically send the electronic file to the customer.