The Pharmaceutical industry is changing quickly and as a wholesale distributor, you must be prepared for specific requirements before the November 2023 deadline that was set out by the DSCSA. As manufacturers, distributors, and software providers prepare, a lot of questions are raised to help us understand why we need to make certain changes. If you understand why the requirements are put in place, you can then find software that has the ability to help you reach those requirements – getting ahead of the game. The big question we are going to answer here is why we need to track lot and serial numbers if we are already tracking lot numbers that tell us so much. Well, to answer this question we first need to understand what lot and serial numbers are and what information they hold.
On top of understanding what those numbers mean, we also have to understand why this change is coming into effect. I’m sure you’ve heard of the opioid crisis – the misuse and addiction to opioids (or controlled substances), which is a serious national crisis that affects public health. While the opioid crisis is not the only reason, it is one of the drivers of change. The ultimate goal of these changes is to be able to manage all information and movement of a single product throughout the supply chain. This will include information about who the manufacturer is, all the way down to the pharmacy that is going to sell product to the consumer.
Review all the guidance’s under the DSCSA released by the FDA
What Is a Lot Number?
A lot number is a code that identifies one batch of a product that is made at the same time. The Food and Pharmaceutical industries are good examples of those who track lot numbers since lot numbers will be associated with additional information such as the expiration date. The purpose of a lot number is to be able to identify one batch of product including expiration but it also allows us to track the Transaction History of the product including who the manufacturer is. In the event where a product is suspect or is considered to have something wrong with it, the batch that the product came from can be tracked using the lot number back to the company who manufactured it - this is considered a “Product Recall”.
What Is a Serial Number?
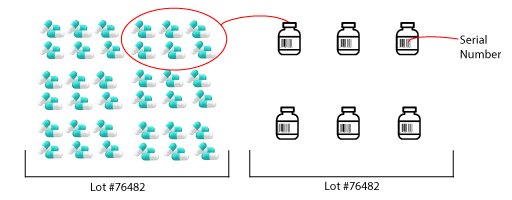
While a lot number identifies an entire batch of product that was made, a serial number identifies the lowest sellable unit. The lowest sellable unit is how the manufacturer decides they want to sell that specific product in - this could be a case or a bottle for example. So, if we look at a medication like aspirin, the lowest sellable unit is likely a bottle of 100 pills. The graphic below shows us that no two bottles will ever share the same serial number, but they may share the same lot number. The purpose of a serial number is to be able to track each individual product or the lowest sellable unit.
As you can imagine, a real manufacturer is making a lot more products than what you see above and because they are making so much in one batch, we know that the entire batch is not going to go to the same end pharmacy or store – they likely will not be able to sell them all before the expiration date. Instead, the batch is separated and distributed to different players. One of the biggest advantages of serialization is the ability to track each sellable unit to all players who have owned the product.
Download our Pharmaceutical Software Buying Guide to help you determine the best software for your business.
Why Do We Have to Track Lot Numbers and Serial Numbers?
The DSCSA’s role is to provide requirements that will help to improve the traceability of products moving through the supply chain. Currently, as products are moving from owner to owner, it is simple to inject an illegitimate product and make it appear as though it is a part of a legitimate batch. Once these products are thrown into the mix there is no way for anyone to track where that one product came from or how many were injected into that batch. Since all the bottles can have the same general look, it now becomes necessary to track more than just the number of units – we need to track each unit. The DSCSA has implemented the requirement for serial numbers because it allows us to identify the subsets of each batch. With serialization, we will be able to track the movement of each individual sellable unit, making it difficult to inject illegitimate or suspect products into the supply chain for misuse.
The November 2023 DSCSA deadline requirements outline more than just the ability to track lot and serial numbers. We strongly encourage that you and your team stay up to date on all requirements.
If you're interesting in learning more about the Pharmaceutical Industry, Blue Link's Darren Myher was interviewed by WBSRocks (a Podcast for growing businesses) titled: “Grow Your Business by Understanding the Unique Nuances of the Pharma Distribution Business w/ Darren Myher”. The Podcast is available on all streaming platforms. You can also access the podcast through any of these links: