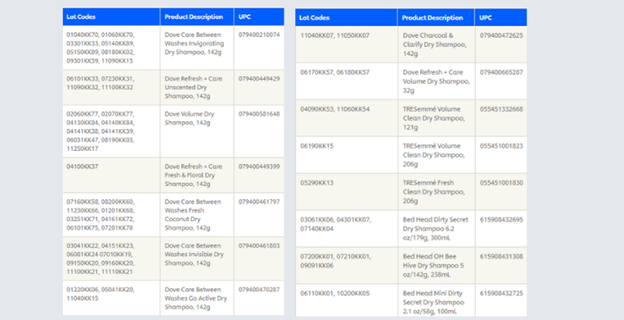
Did you know that approximately 220 recall incidents are overseen by The Canadian Food Inspection Agency (CFIA) every single year? Do you realize that this is just food recalls? This past month alone there have been two recalls in Canada by large distributor Unilever and a group of Pine-Sol products. The Unilever recall includes popular aerosol dry shampoo products from big brands such as Dove, TRESemmè, and Bed Head for items sold between January 2020 and October 2022 due to potentially high levels of benzene. Benzene is a colorless or light-yellow chemical that is highly flammable. People who breathe in high levels of benzene may develop signs and symptoms including drowsiness, headaches, and confusion within minutes to several hours.
Unilever has been able to identify exactly which products need to be taken off sale floors (or be discarded by the consumer) through the identification and tracking of lot codes. Applying lot codes to groups or batches of products provides valuable information to businesses wanting to know inventory information or dealing with a product recall.
Consumer goods, toys, prescription drugs or medical devices, and food are all susceptible to recalls due to safety or quality concerns relating to manufacturing or the design/packaging of the products. The information that needs to be tracked includes dates, origin, location, and weight. It just goes to show the importance of having lot tracking software for any distributor.
Product Recalls
Product recall is a harsh reality and an inconvenience that businesses need to face and deal with in a swift manner. It not only costs the company time and money to track item purchases and sales, but it can bring an entire company to a standstill. Worse, it can harm the company’s reputation if not dealt with in a timely, efficient and appropriate fashion. The speed with which a recall is handled can tilt the balance of customer satisfaction (up or down), which ultimately affects the company’s bottom line.
Efficiency and speed are essential for effectively handling product recalls as the company’s reputation and liability may be riding on a successful outcome. The best choices and processes are those that reduce the time it takes to recall the items. Relying on a paper trail is no longer acceptable as it is prone to human errors such as loss of papers and incorrect information.
The best business tracking tools include effective software, trained employees and established business processes. Investing in a clear and logical business process, employee training and the right ERP lot tracking software results in confident employees that can deal with the recall in the best possible manner –a worthwhile investment which may eventually save your company from a downturn or worse.
Pharmaceutical Specific Software for Product Tracking
As you can imagine, the pharmaceutical industry is characterized by many regulations and an abundance of meticulous standards that must be met by suppliers and distributors. As an increasing number of products enter the marketplace, the need for tight control measures becomes more prevalent in order to ensure public health and safety – hence the introduction of the DSCSA. The onus lies with the suppliers and distributors to meet (perhaps exceed) the standards in place.
Learn about a distributors responsibilities regarding the DSCSA.
However, despite these standards, certain circumstances have arisen where products have needed to be recalled in the interest of public health. Some of the most prominent drug recalls in terms of scale and magnitude include:
(1) Fenfluramine/Phentermine (Fen-Phen) – developed by Wyeth-Ayerest Labs was recalled in 1997 after 24 years on the market. Fen-Phen was a popular weight loss drug taken by approximately 6.5 million people to help combat obesity. After many users began experiencing heart disease and other related illnesses, the FDA set a recall in motion. The result was roughly $14 billion in damages paid to victims.
(2) Cerivastatin (Baycol) – developed by Bayer and was prescribed to patients as a treatment for high cholesterol and was later linked to a severe muscle disorder. It was recalled in 2001, after roughly 4 years in the market. Damages paid to victims totalled roughly $1.2 billion.
(3) Rofecoxib (Vioxx) – widely considered to be the largest drug recall in history, took place in 2004 after 5 years on the market. Vioxx was prescribed to more than 20 million people as a pain reliever for arthritis; unfortunately, it was found to be responsible for increased risk of heart attack and stroke. Evidence suggests that nearly 140,000 people could have suffered from serious heart conditions as a result.
Visit this website for more information on these recalls and others in the industry.
Despite the standards in place and the sheer amount of testing and preparation involved in bringing a product to market, situations like those above may still arise. In the first example, it wasn’t until nearly a quarter of a century later that complications were discovered. As a result, a growing number of distributors have turned to ERP vendors to provide a system that can not only be fully integrated into their everyday business operations but also provide flexibility to meet pharmaceutical industry-specific requirements and the ability to act promptly in dire circumstances. Some of these features include:
DEA & State License Expiry Management
Effective ERP systems can successfully identify the type of license, state, license number, expiry date, and allowable drug schedule for each customer in the system. This information needs to be easily accessible to provide users with the ability to generate reports about licenses approaching expiry.
Pedigree Management
Adhering to FDA standards, pharmaceutical distributors need a system that includes pedigree management. A drug pedigree is a statement of origin that identifies each prior sale, purchase, or trade of a drug, including the date of those transactions and the names and addresses of all parties. The pharmaceutical distribution system selected by your company needs to provide the ability to enter information about a specific drug’s route from the manufacturer to your company.
These are just a few specific features that must be considered when deciding specifically on pharmaceutical distribution software platforms. In addition to the industry specific features available, the system should also be able to handle a company’s basic inventory, accounting, contact management, and order entry processes.
This will ensure you implement an all-encompassing solution that will meet your basic business needs as well as the numerous industry requirements and regulations. Should there be an extenuating circumstance where you are required to recall certain products, pharmaceutical distribution software will provide your company with the necessary tools to facilitate that response in a timely and effective manner.
Blue Link ERP Software with Lot Tracking Functionality
Blue Link ERP Includes lot tracking functionality as part of our all-in-one ERP Software so that small-medium sized wholesale distributors don’t have to worry about manually tracking products even after a sale. Recalls are a prime example of the importance of lot tracking; even 2 years after a sale, the manufacturer, retailer, or anyone for that matter might find an issue with the product that can result in a full product recall nationwide. Distribution companies around the world are responsible in part for the safety of the communities their products are being sold to so these recalls need to be dealt with the highest level of importance. The only way to accurately track these products after the sale is through lot codes.
Some distributors don’t bother tracking lots at all because they know that manually managing and record keeping is time consuming and prone to human error. Having lot tracking software as part of your base software application can make – or break – a company. The product recall process is completely seamless for you and best of all, with minimal downtime. Product recalls are a tough reality and when you are faced with a recall, the speed which the recall is handled can either increase or decrease overall customer satisfaction. With Blue Link’s automated lot tracking, after a quick search, you will be able to generate a report of the customers who received the specific product and contact them immediately to take appropriate action.
The Blue Link’s lot tracking functionality includes sophisticated features often only found in much more expensive software. It is well designed with the ability to access and retrieve all relevant information and traces finished goods throughout the distribution chain, from the sales order through to the customer. Automated traceability allows you to track product both on company premises and even after the sale when you are no longer holding a product. Even if the product is sitting on a shelf in a retail store, you will be able to track it.
Especially during a product recall, it is crucial that the business has accurate product information through storing lot number(s), bin and shelf location, date, weight and origin etc. This information is also beneficial for a customer lot inquiry or an internal inventory count. This allows you to quickly look up product information in the system, without having to manually sort through files or find product on your warehouse shelves. For further efficiencies, Blue Link ERP allows you to use different lot numbers other than the manufacturer’s number. Theirs can be assigned as the external lot number or a completely new lot number can be created internally. If an internal lot number is required, this can be done through auto-generation or by a user-assigned number system.
Our capabilities are used by a variety of industries and for multiple purposes including product traceability, basic Return Merchandise Authorization (RMA) and increased inventory management. It also allows you to perform lot costing which means you can track actual costs instead of using the FIFO Method or Average Costing. If you want to understand the differences between Lot Costing, FIFO and Average Costing, watch the video below.

 Click to enlarge
Click to enlarge








